Abstract
Introduction: Sequential Hodgkin lymphoma (HL) trials of the Children's Oncology Group (COG) have tested treatment strategies intended to reduce late toxicity while maintaining high rates of cure. However, the long latency of the relevant late effects makes it impossible to observe in a timely way the degree to which modifications to protocol-directed treatment will achieve this goal. The purpose of this study was to estimate late cardiac disease risk associated with sequential COG trial protocols using established risk models.
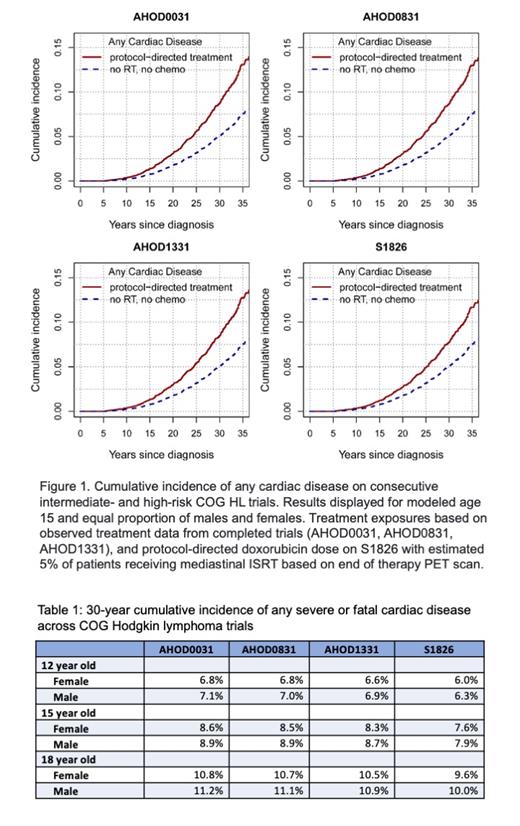
Methods: We estimated the late severe or fatal cardiac toxicity associated with three consecutive completed COG trials enrolling intermediate- and high-risk classical HL patients: AHOD0031 (2002-2009), AHOD0831 (2009-2012), and AHOD1331 (2015-2019). Patients were less than 22 years old at the time of trial enrollment. In addition, we applied the model to the ongoing S1826 trial protocol for patients ≥12 to <22 years of age. Eligible disease stages were IB, IAE, IIB, IIAE, IIIA, IVA with or without bulk disease and IA or IIA with bulk disease for AHOD0031; IIIB and IVB for AHOD0831; IIB with bulk, IIB, IVA and IVB for AHOD1331; and III and IV for S1826. When radiotherapy (RT) was indicated, prescribed RT was 21Gy involved-field radiotherapy (IFRT) in AHOD0031, 21Gy IFRT in AHOD0831, 21-30Gy involved-site radiotherapy (ISRT) in AHOD1331, and 30-36Gy ISRT in S1826. Cardiac toxicity included coronary artery disease, heart failure, valvular disease, pericardial disease and arrhythmias. Statistical models quantifying the association between age, sex, doxorubicin dose, alkylating agent exposure and cardiac radiation dose and late cardiac risk were derived from HL survivors in the Childhood Cancer Survivor Study (CCSS) (Bates 2019 J Clin Oncol 1;37(13):1090-1101 & Shrestha 2021 Radiother Oncol, in press). These demographic and dose-risk relationships were then applied to the delivered COG trial protocol exposures to predict the 30-year cumulative incidence (CI) of cardiac disease for trial patients. To estimate the average cardiac risk, we accounted for the chance of receiving mediastinal RT, mean cardiac radiation dose among those receiving mediastinal RT, and cumulative doxorubicin dose, according to respective trial data.
Results: Based on the median age of enrolled patients, results are reported for modeled age 15 years at treatment. The cumulative planned doxorubicin dose was 200mg/m 2 on AHOD0031 and AHOD0831, and increased to 250mg/m 2 on AHOD1331 to improve progression-free survival compared to the prior trials for select groups of higher risk patients. Doxorubicin dose was 300mg/m 2 on S1826 to limit RT use to selected patients with persistent disease on positron emission tomography (PET) at the end of systemic therapy. Mediastinal RT was given to 62% of patients on AHOD0031 (mean heart dose 13.2Gy), 58% on AHOD0831 (mean heart dose 13.6Gy), and 53% on AHOD1331 (mean heart dose 10Gy). For S1826, we assumed a 5% rate of mediastinal RT use, mean heart dose of 10Gy, and no long term cardiac risk associated with nivolumab or brentuximab. For the average 15-year old patient, the predicted 30-year cumulative incidence of any cardiac disease was 8.8% in AHOD0031, 8.7% in AHOD0831, and 8.5% in AHOD1331 (Figure 1). Despite the increase in doxorubicin dose, the anticipated reduction in the utilization of mediastinal RT in S1826 is predicted to reduce the 30-year risk of cardiac disease to 7.8%. The predicted 30-year cumulative incidence of any cardiac disease, for the average 12-, 15-, and 18-year old males and females are shown in Table 1.
Conclusions: From 2002 - 2019, reductions in the proportion of patients receiving mediastinal RT and smaller target volumes with ISRT produce lower cardiac radiation dose, which is predicted to offset increase in doxorubicin dose and produce a small reduction in late cardiac disease. Limiting RT use based on end of chemotherapy PET on S1826 is predicted to further reduce late cardiac disease among children with HL risk despite higher doxorubicin dose in this protocol. Increasing use of dexrazoxane may further mitigate risk for cardiac toxicity in more contemporary cohorts. Clarification of the impact of nivolumab, brentuximab and relapse therapy would allow for a more complete prediction of cardiac disease risk.
Herrera: Bristol Myers Squibb: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Gilead Sciences: Research Funding; Genentech: Consultancy, Research Funding; Kite, a Gilead Company: Research Funding; Tubulis: Consultancy; Takeda: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Seagen: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Karyopharm: Consultancy. Friedberg: Acerta: Other: DSMC ; Bayer: Other: DSMC ; Novartis: Other: DSMC .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal